Published by: Zaya
Published date: 25 Jun 2021
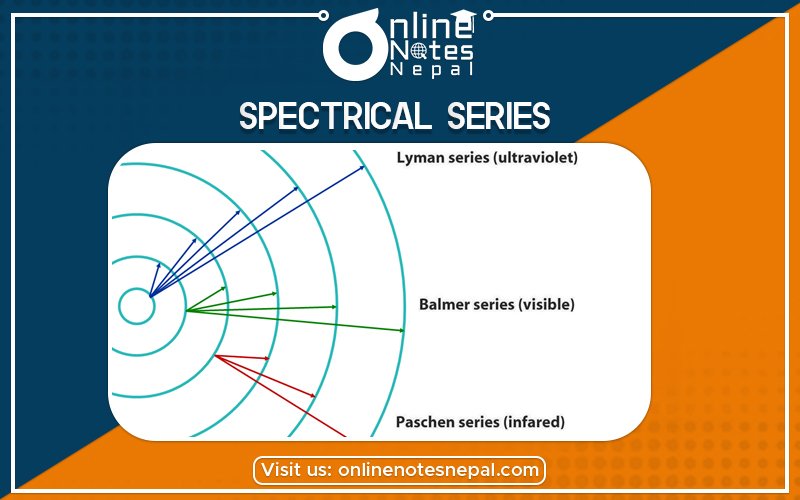
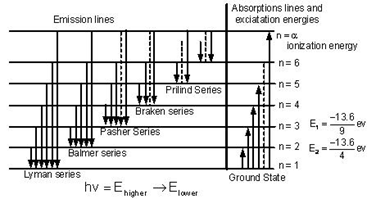
When electrons move around the nucleus in certainly permitted orbit, it can jump from higher to lower energy level with the emission of radiation. The wavelength of emitted radiation can be obtained by the expression:

Where R2= Rydberg constant
n1= lower energy level
n2 = higher energy level
On the basis of the transition of an electron from higher to lower energy level different spectral series can be obtained which are explained below:
Lyman series:
If the transition of electron is to n1= 1 from n2 = 2,3,4…. Lyman series can be obtained. The wavelength of the layman series can be obtained as

The wavelength lies in the region of the ultraviolet.
Balmer Series:
If the transition of electron is to n1=2 from n2 = 3,4,5… Balmer series is obtained. The wavelength of the Balmer series can be obtained as

The wavelength lies in the visible region.
Paschen series:
If the transition of electrons is to n1 = 3 from n2 = 3,5,6 …. Paschen series can be obtained. The wavelength of the Paschen series is given by

The wavelength lies in the infrared region.
Brackett series:
If the transition of an electron is to n1= 4 from n2 = 5, 6,7 …. bracket series is obtained. The wavelength of the Brackett series is given by

The wavelength lies in the infrared region
Pfund series:
If the transition of electrons is to n1 = 5 from n2 = 6 ,7,8 … Pfund series is obtained. The wavelength of the Pfund series is given by

The wavelength lies in the infrared region