Published by: Nuru
Published date: 16 Jan 2022
Symbol: O
Molecular formula: O
Valency: 2
Position in periodic table: Group-VI A, Period-2nd
Electronic configuration: 2, 6 (1s2, 2S2 2P4)
Atomic number: 8
Atomic weight: 16
Molecular weight: 32 amu
Freezing point:-219°C
Boiling point: -183°C
Oxygen is abundantly occurring element on the earth. It constitutes about 47.6% of the earth's crust.
General methods for the preparation of oxygen gas
a. From metallic oxides:
Oxides of metals give oxygen when heated.
2HgO → 2Hg + O₂↑
b. From metal peroxide:
When sodium peroxide is treated with water, oxygen is liberated.
2Na₂O₂ + 2H₂O → 4NaOH + O₂↑
c. From electrolysis of water:
Oxygen is evolved in the positive electrode during the electrolysis of acidic water in Hofmann Voltameter.
2H₂O→ 2H₂ +O₂
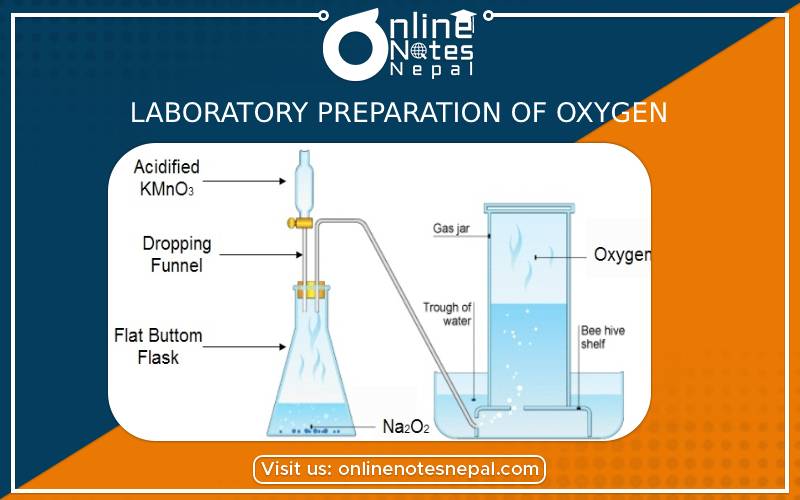
Oxygen gas is prepared in laboratory by the following two methods:
Principle:
When potassium chlorate is heated in the presence of manganese dioxide in the ratio of 3:1, it decomposes at 250°C into potassium chloride and oxygen.
2KClO₃ → 2KCl + 3O₂
Take a mixture of powdered potassium chlorate and manganese dioxide in the ratio of 3:1 in a hard glass test tube. Fit it with a cork and a delivery tube and adjust the test-tube in a stand as shown in the figure. Insert the other end of delivery tube into the bee-hive shelf. A gas jar is filled with water and inverted over the bee-hive shelf in the pneumatic trough. Now, heat the mixture. When potassium chlorate is decomposed, oxygen is liberated.
The first formed oxygen gas is contaminated with the air inside the hard glass test tube and delivery tube and is allowed to escape. Then the oxygen is collected in gas jar by downward displacement of water.
Precautions
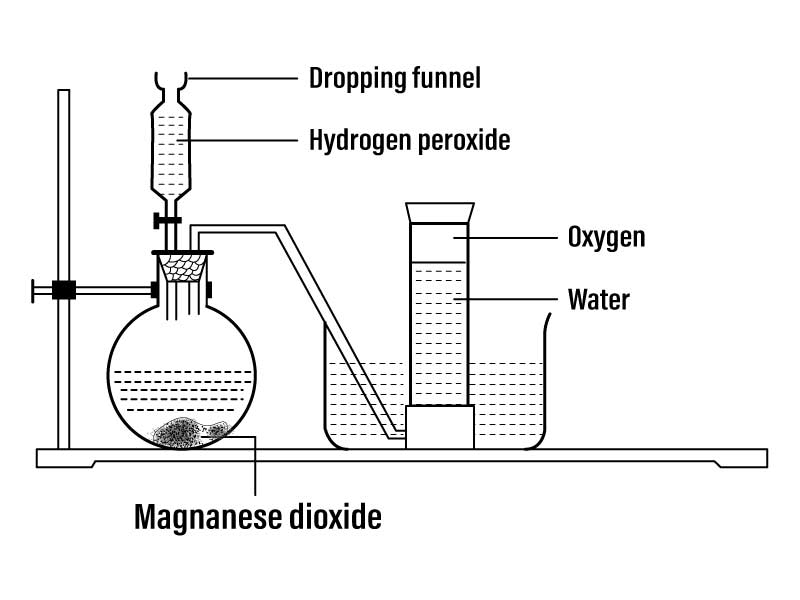
Principle:
Oxygen gas can be prepared in the laboratory by hydrogen peroxide and manganese dioxide. In this reaction manganese dioxide is used as catalyst.
2H₂O₂→ 2H₂O + O₂↑
The apparatus is set up as shown in the figure. Hydrogen peroxide is poured in manganese dioxide and water having a conical flask with the help of a thistle funnel. Inside the flask, the decomposition of hydrogen peroxide is taking place in the presence of manganese dioxide and oxygen gas is liberated.
The produced oxygen gas is collected in the gas jar by downward displacement of water.
Test of oxygen:
To test whether the produced gas is oxygen or not, introduce a glowing matchstick in the jar containing gas. This burns with bright light.
a. From air:
By compression, cooling and sudden expansion of air, the air is changed into liquid state and is freed from moisture and carbon dioxide. The liquid air has nitrogen and oxygen only. The liquid air allowed to vaporize. Being the boiling point of nitrogen lower, it escapes first and is separately collected. The left liquid is nearly pure oxygen.
b. By electrolysis of water:
Oxygen can also be manufactured by the electrolysis of water as you are explained in hydrogen gas.
Physical properties
Chemical properties