Published by: Nuru
Published date: 16 Jan 2022
Symbol: N
Molecular formula: N₂
Valency: 3
Position in periodic table: Group-V A, Period-2nd
Electronic configuration: 2, 5 (1S₂, 2S₂ 2P₃)
Atomic number: 7
Atomic weight: 14
Molecular weight: 28 amu
Freezing point:- 210 °C
Boiling point: -196 °C
Nitrogen is found in both free and combined states. It occupies 78.07% by volume.
A. By heating copper in air:
Firstly, air is passed through lime water and then concentrated sulphuric acid to make the air free from carbon dioxide and moistures respectively. The air contains only nitrogen and oxygen which are passed over the heated copper wire as shown in the figure. Here copper combines with oxygen to make copper oxide and nitrogen gas passes over. This nitrogen can be collected by downward displacement of water.
B. By burning phosphorous in air:
Nitrogen gas can be prepared by burning phosphorous in air. A crucible with phosphorous is taken and allowed to float on water. Then phosphorous is burnt in crucible is covered with gas jar as shown in figure. The oxygen of the gas jar is consumed during the burning of phosphorous penta oxide is formed. The gas left in the jar is nitrogen.
4P + 5O2 → 2P2O5
C. By decomposition of compounds containing nitrogen
Like, when dilute solution of ammonium nitrite is heated, nitrogen gas is produced.
NH₄NO₂→ N₂ +2H₂O
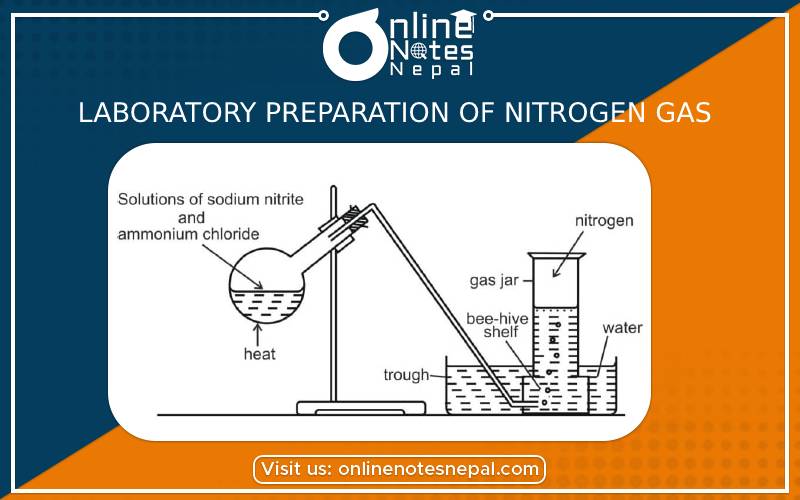
Principle:
When the solution of sodium nitrite and ammonium chloride is heated they react together and liberate nitrogen gas.
NaNO₂ + NH₄Cl → NaCl + H₂O +N₂
Take a mixture of ammonium Chloride and Sodium nitrate in equivalent amount in a round bottom flask. Some water is added to make the solution of the mixture. Then the delivery tube is fitted with a round bottom flask with the help of a cork. The flask is heated gently with a source of heat. After that gas is produced by the reaction of chemicals and is collected in gas jar by downward displacement of water.
Precautions are as follows:
Test of nitrogen are as follows:
When burning magnesium ribbon is inserted in a jar containing gas, if it burns continuously and forms magnesium nitride, it proves that gas is nitrogen.
Physical properties
Chemical properties