Published by: Nuru
Published date: 16 Jan 2022
Hydrogen
The thick layer of air which surrounds the earth is called atmosphere.
Composition of air
| S.N | Gases in air | Percentage by volume |
| 1 | Nitrogen | 78.07 |
| 2 | Oxygen | 20.98 |
| 3 | Carbon dioxide | 0.03 |
| 4 | Argon | 0.85 |
| 5 | Neon | 0.002 |
| 6 | Other inert gases | 0.008 |
| 7 | Water vapor | 0.06 |
Symbol: H
Molecular formula: H2
Valency: 1
Position in periodic table: Group-1A, Period-1st
Electronic configuration: 1 (1s1)
Atomic number: 1
Atomic weight: 1.008
Molecular weight: 2.026amu
Freezing point: -259.14 °C
Boiling point: -252.87 °C
Occurrence
Hydrogen is a reactive element and thus does not occur much in the free state. In combined form, it is an important constituent of water, acid, alkali, and many organic compounds of vegetables and animal products.
a. From acids:
Metals like zinc, iron, magnesium, etc. are more electropositive than hydrogen and react with acid to produce hydrogen gas.
Zn + dil. H2SO4 → ZnSO4 +H2
b. From alkalis:
Hydrogen gas can be obtained from the action of metals like zinc, aluminum etc. with boiling caustic soda.
Zn + NaOH → Na2ZnO2 + H2↑
From water: At ordinary temperature, highly active metals like sodium, potassium, calcium, etc. react with water to liberate hydrogen gas.
2Na + 2H2O → NaOH + H2↑
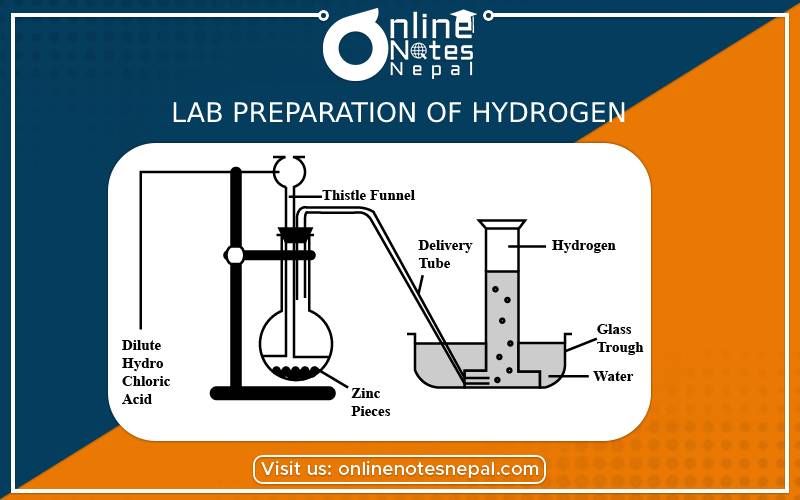
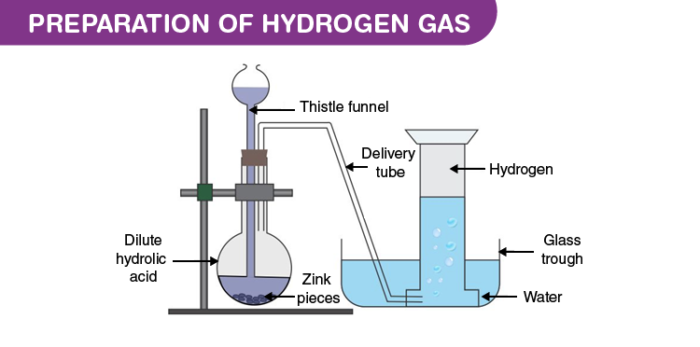
Principle:
When impure granulated zinc reacts with dilute sulphuric acid. They react together to form zinc sulphate and hydrogen gas. The principle reaction is as follows.
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen↑
Zn + dil. H₂SO4 → ZnSO4 + H₂
Take a few grains of granulated zinc in Woulfe’s bottle fitted with a thistle funnel and delivery tube with corks. Put the next end of the delivery tube under water in pneumatic trough having a beehive shelf. Invert a gas jar, completely filled with water over the beehive shelf and let the end of the delivery tube into it. Pour dilute sulphuric acid through the thistle funnel. A brisk action sets in and hydrogen gas is evolved.
Then hydrogen gas gets collected in the gas jar by downward displacement of water.
Zn + dil. H₂SO4 → ZnSO4 + H₂
If the reaction is slow then we can add a little copper sulphate solution to accelerate the reaction.
Precaution are as follows:
Test of hydrogen:
To check whether the produced gas is hydrogen or not, when lighted splinter is introduced to the mouth of gas jar. The gas burs itself with a very faint pale-blue flame at the mouth of jar with pop sound and the splinter gets extinguishes.
a. From methane-steam process:
When a mixture of steam and methane is passed over a heated nickel catalyst at 1200 ͦC, and compressed to 30 atmospheres, Hydrogen gas is manufactured. Methane is obtained as a byproduct of petroleum industry.
CH₄ + H₂O → CO + 3 H₂
A catalyst is as a chemical substance which alters the rate of chemical reaction, itself remaining chemically unchanged because it does not take part in chemical reaction. The phenomenon is known as catalysis.
b. By the electrolysis of water:
A small amount of dilute acid is poured into a voltameter containing water to make a strong electrolyte as shown in the figure. In the electrolytic cell or voltameter iron is used as cathode while nickel is used as cathode while the nickel-plated iron acts as anode. An asbestos diaphragm separates these two electrodes from each other. This diaphragm prevents the mixing of hydrogen gas and oxygen gas. When electric current is passed, hydrogen is formed at cathode and oxygen at anode.