Published by: BhumiRaj Timalsina
Published date: 22 Jun 2021
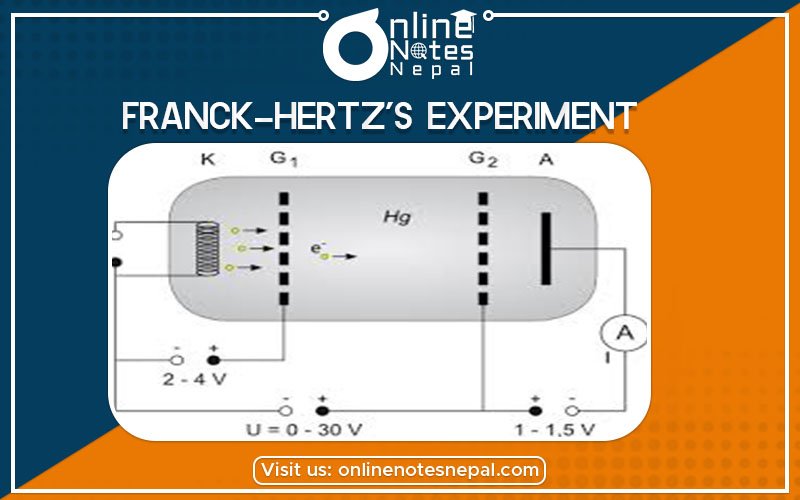
Franck-Hertz’s experiment, first undertaken shortly after Bohr’s theory of the atom was presented, provided one of the early indications that atoms had discrete energy levels. In Franck-Hertz’s experiment, electrons are accelerated and passed through mercury vapor, where they lose energy by
inelastic scattering in quantized steps as they excite mercury atoms from the ground state to an excited state. This elegant experiment yielded remarkable results that were key in the early developments of quantum theory.
In the Franck-Hertz’s experiment, an electron beam is produced by thermionic emission from a filament. The electrons are accelerated, pass through the vapor, and are then retarded (decelerated) by a few volts before collection at the anode. This all takes place in a tube contained within an oven that controls the tube’s temperature and thus the mercury vapor density.
Picture
Consider first what happens as the filament is heated, thus raising the average energy of conduction electrons in the metal. As the temperature increases, a greater fraction of the electrons have kinetic energy exceeding the work function of the cathode material (platinum), WPt. Some of these even reach the anode and a small current (e.g., nanoamps) flows. When the accelerating voltage Va is increased from zero, filament electrons are accelerated toward the grid and a much greater current reaches the anode. If Vg= 0, some of the emitted electrons reach the anode and the electron current is registered. The path of current in this circuit passes through the two power supplies, the filament, the mercury vapor in the tube, and through the ammeter. Not all the electrons make it to the anode: the “beam” really isn’t a beam, and the trajectories spread out over a large range of angles. The planar geometry does improve the situation. As the accelerating voltage increases, the anode current increases because the electron trajectories are more focused, and the electrons are deflected less by scattering from the atoms of vapor in the tube. The anode current is observed to rise faster than linearly with Va.
We now take into account electron collisions with the mercury atoms in the vapor. Elastic scattering is one channel that results in the deflection of the electrons from their original trajectory. In an elastic scattering, the atom “recoils” in its ground state like a hard-sphere, so the electron loses very little energy. Inelastic scattering is also possible. In this process, kinetic energy is lost to excitation or ionization of mercury atoms. The excitation energy is Eex=5eV, so, when (eVa-WPt) < Eex, no such excitation is possible. For electron kinetic energy greater than Eex, it is, in principle, possible for the inelastic process to occur and decrease the electron kinetic energy by about Eex for each mercury atom an electron excites.
The scattering process is best represented by a cross-section! — the effective area of a mercury atom presented to the electrons as they move through a vapor. Imagine looking through the vapor with total thickness t and a number density n atoms/cm3 from the perspective of an electron with some initial trajectory. The vapor may look partly transparent with an areal density of ntatom/cm2 in the electron’s path. The total fraction of that area taken up by mercury atoms from which the electron can scatter is !nt , a measure of the probability the electron will scatter while propagating through the vapor. In the questions, we ask you to estimate! geometrically and find t 1/ 2, the thickness of vapor that would scatter electrons with 50% probability. The cross-section is an effective area and depends on the nature of the interaction, initial energy, final energy and direction, and other quantum mechanical features of the process. The elastic and inelastic cross sections are generally quite different: the inelastic excitation cross-section can be a resonant process with a relatively large cross-section.
As an electron passes through the vapor, it gains energy in the accelerating field produced by Va. At the point where the electron’s energy exceeds Eex, inelastic scattering becomes possible, and the electron scatters with a short scattering length and loses almost all of its kinetic energy so that the process begins again. Thus multiple inelastic scatterings are possible as an electron moves from cathode to grid. The final energy of electrons that reach the grid is the initial energy, eVa, minus energy lost in scatterings. A small retarding voltage between the grid and anode deflects electrons with energy less than eVg+WCu, where WCu is the work function of the anode. This energy is a few eV. Electrons with greater energy reach the anode and the electron current is registered by the ammeter. For electrons that lose total energy !E by scattering, the requirement for detection (reaching the anode) is !E < e Va”Vg ( ” !W ), where !W , the “contact potential”, is the difference of work functions of the cathode and anode. The anode current is therefore a measure of the integral number of electrons that satisfy this inequality. These raw data must be analyzed to determine the electron energy loss spectrum and extract Eex.
If you liked our content Boolean Algebra, then please don’t forget to check our other topics Program Design.