Published by: Nuru
Published date: 26 Jun 2021
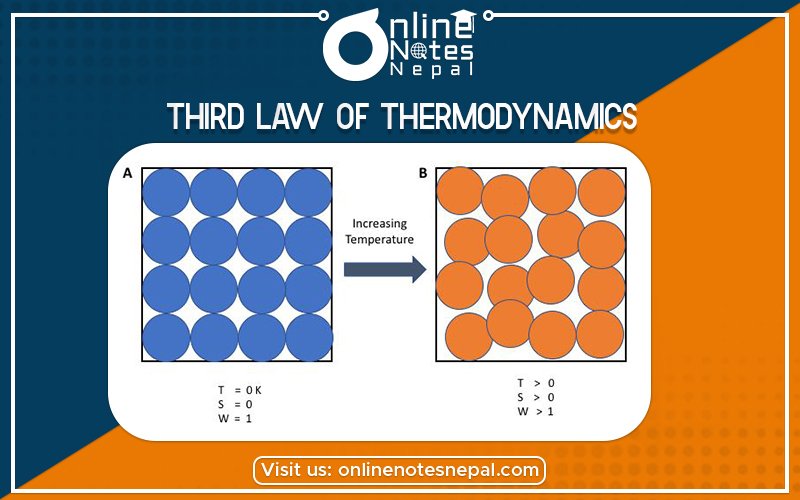
The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero. Most thermodynamics calculations use only entropy differences, so the zero points of the entropy scale are often not important. However, we discuss the Third Law for purposes of completeness because it describes the condition of zero entropy.
The Third Law states, “The entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute zero (0 K).”
It is the lesser-known of the three major thermodynamic laws. Together, these laws help form the foundations of modern science. The laws of thermodynamics are absolute physical laws – everything in the observable universe is subject to them. Like time or gravity, nothing in the universe is exempt from these laws. In its simplest form, the Third Law of Thermodynamics relates the entropy (randomness) of matter to its absolute temperature.
The Third Law refers to a state known as “absolute zero.” This is the bottom point on the Kelvin temperature scale. The Kelvin scale is absolute, meaning 0° Kelvin is mathematically the lowest possible temperature in the universe. This corresponds to about -273.15° Celsius or -459.7 Fahrenheit.