Published by: Nuru
Published date: 27 Jun 2021
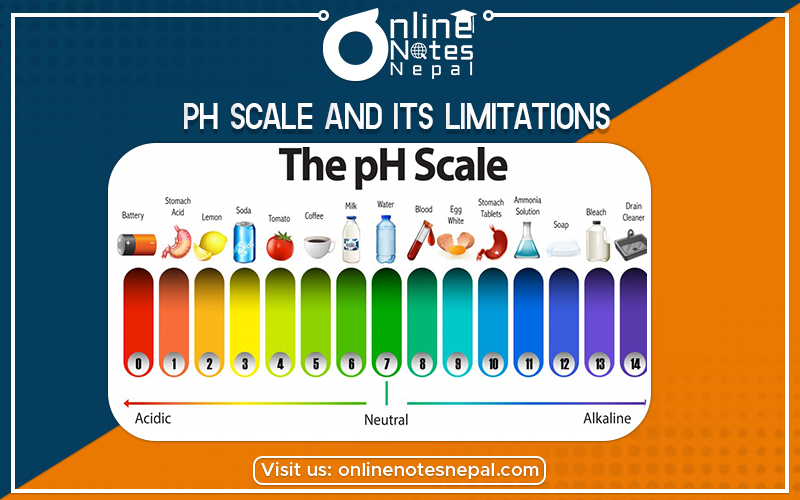
Limitations of pH Scale- (i) pH values of the solutions do not give us an immediate idea of the relative strengths of the solutions.
pH is an important quantity that reflects the chemical conditions of a solution. The scale which is used to measure the acidity or alkalinity of a given solution is called the PH– Scale. The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. Numbers less than ‘7’ i.e. 6,5,4… indicate an increase in acidity while numbers more than 7 i.e. 8,9,10… indicate the increase in alkalinity of the solution.
Greater the value of PH of a solution lower will be its hydrogen ions concentration as hence, weaker is the acid. In the case of the acidic solution, the PH value increases after dilution while in the case of alkaline solution the PH decreases after dilution.
Hence, A pH value is a number from 1 to 14, with 7 as the middle (neutral) point. Values below 7 indicate acidity which increases as the number decreases, 1 being the most acidic.
Therefore the pH scale and its limitations are explained above.