Published by: Nuru
Published date: 26 Jun 2021
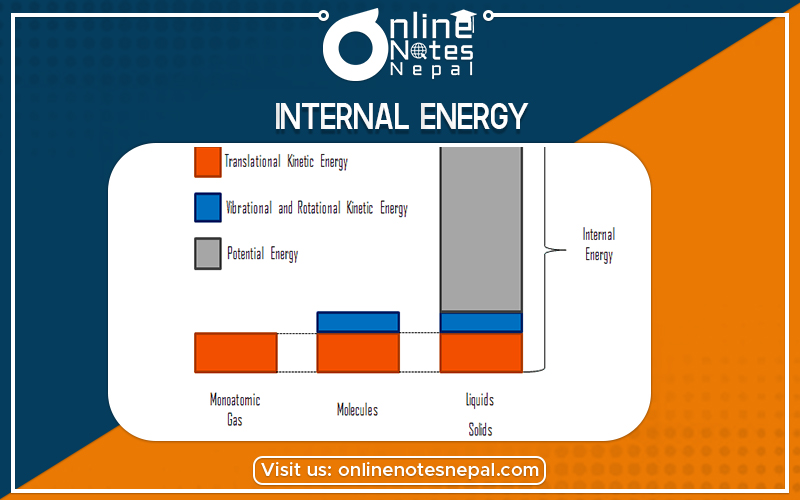
In thermodynamics, the internal energy of a system is the energy contained within the system. It is the energy necessary to create or prepare the system in any given state but does not include kinetic energy. of motion of the system as a whole, nor the potential energy of the system as a whole due to external force fields which include the energy of displacement of the system’s surroundings. It keeps account of the gains and losses of the energy of the system that are due to changes in its internal state.
It is a system that can be increased by the introduction of matter, by heat, or by doing thermodynamics work on the system. When matter transfer is prevented by impermeable containing walls, the system is said to be closed and the first law of thermodynamics defines the change in internal energy is the sum of the heat added to the system and the thermodynamic work done by the surroundings on the system. If the containing walls pass neither matter nor energy, the system is said to be isolated and its internal energy cannot change.
The energy of the constituent particles because their magnitude depends upon the state of the system but does not depends upon the path by which it has been reached.
Suppose E, is the initial state and E2, is the initial energy of a system in the final state then the change in initial energy i.e. ΔE =EP – ER.
Similarly, if ER be the initial energy of the reactant and Ep be the initial energy of the product then the change in internal energy i.e. ΔE= EP – ER.