Published by: Nuru
Published date: 26 Jun 2021
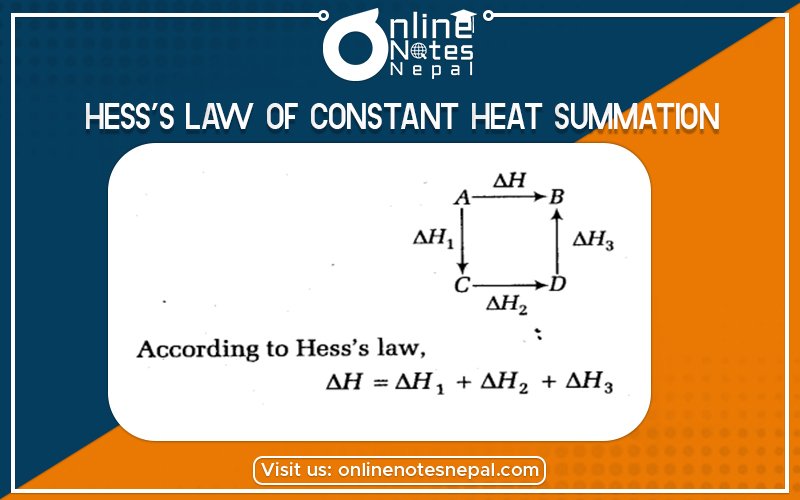
Hess’s law of constant heat summation is the amount of heat evolved or absorbed in a given chemical reaction is always the same whatever the process completes in one step or in a series of steps.
Let us consider a substance A is directly converted to Z in one step.
A→ Z+Q,
where Q is the amount of heat evolved.
Let us consider that A converted into Z in a series of steps.
A→B+q1
B→C+q2
C→Z+q3
The total amount of heat evolved i.e. Q2= q1+ q2+ q3
According to Hess’s Law.
Q1=Q2
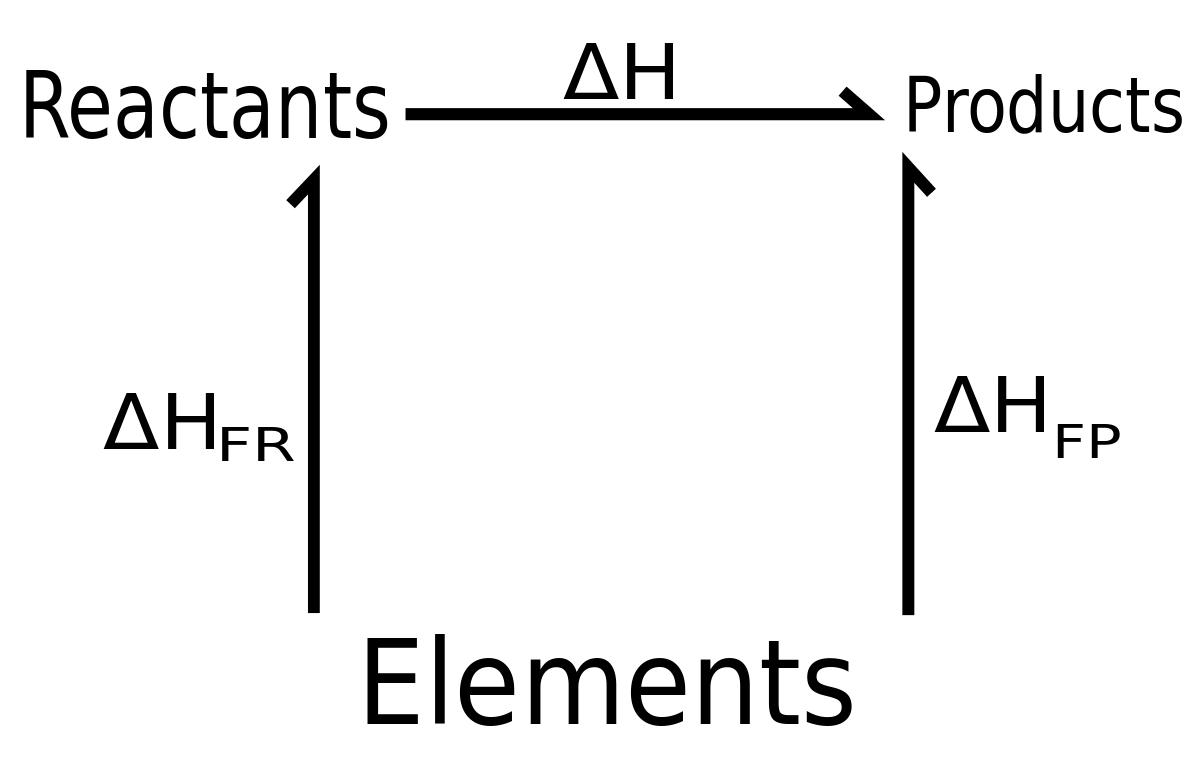
Hess’s Law can also be deduced from the first law of thermodynamics.
Lets us suppose that Q1>Q2 converting
A to Z through the path I heat equals to Q1 is evolved and converting Z to A through path II heat equals to Q2 will be absorbed since Q1>Q2 so in one complete cycle heat equals to Q1– Q2 will be evolved. By repeating this cycle, again and again, a large amount of heat will be evolved which is against the first law of thermodynamics hence Q1 is always equaled to Q2.
Hess’s Law can be illustrated by taking the formation of carbon dioxide from carbon. Carbon is converted into carbon dioxide by two different paths.
1) In the path I carbon is directly converted into carbon dioxide by burning carbon in the presence of an excess of air.
O+C2 → CO2 (g), ΔH= -94 kcal
2) In path II first carbon is burnt in a limited supply of air to get carbon monoxide gas. The carbon monoxide gas is now burnt in excess of air to get carbon dioxide gas.
C+ O2 →CO (g), ΔH= -26 kcal
CO (g) + O2 →CO2 (g), ΔH=-68 kcal
The total amount of heat evolved in path II is -26 -68 i.e. -94 kcal, which is exactly equal to the amount of heat evolved in path I and thus, illustrates Hess’s law of constant heat summation.