Published by: Nuru
Published date: 27 Jun 2021
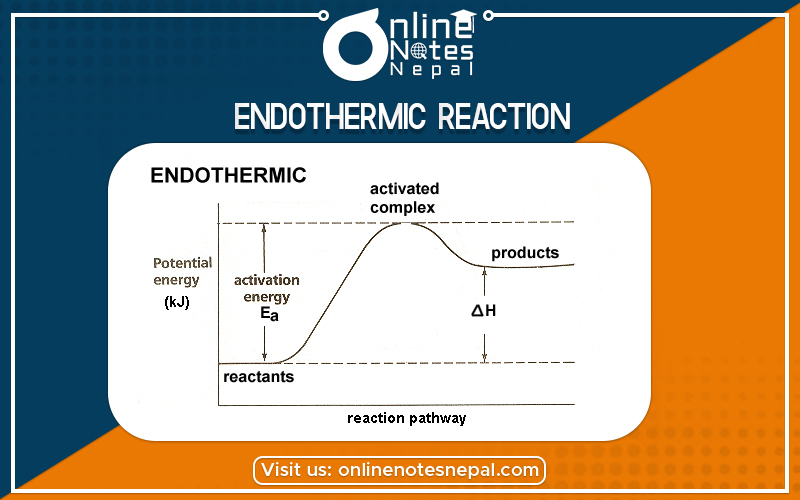
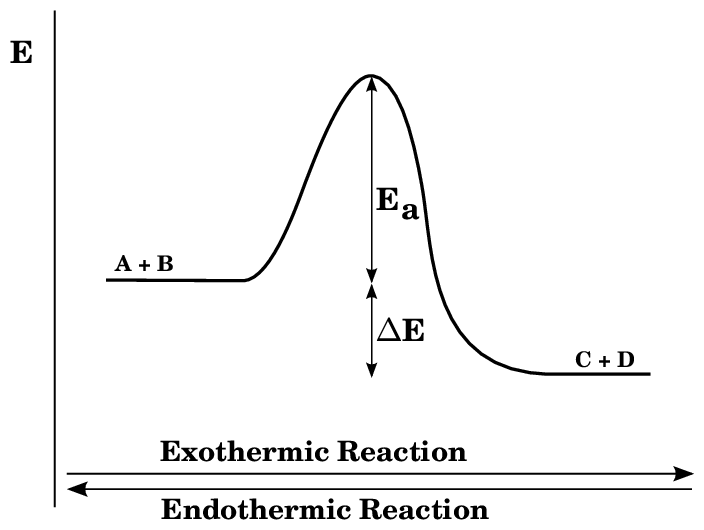
The chemical reaction in which heat is absorbed by the system is called an endothermic reaction. In such type of reaction, ΔH is positive which indicates Enthalpy of product is higher than Enthalpy of reaction.
A good example of this reaction includes dissolving a salt. It doesn’t have to be table salt, nor does the solvent need to be water.
These examples could be written as a chemical reaction, but are more generally considered to be endothermic or heat-absorbing processes:
An endothermic reaction is a type of endergonic reaction. However, not all endothermic reactions are endothermic. Endothermic reactions involve heat absorption. Other forms of energy that might be absorbed in an endergonic reaction include sound and light.
The energy profile diagram for endothermic reaction can be given as:-
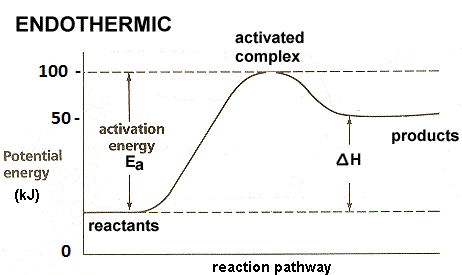
For example
N2(g) + O2(g) ⇌ 2NO(g), ΔH= 180.75 KJ.