Published by: Zaya
Published date: 26 Jun 2021
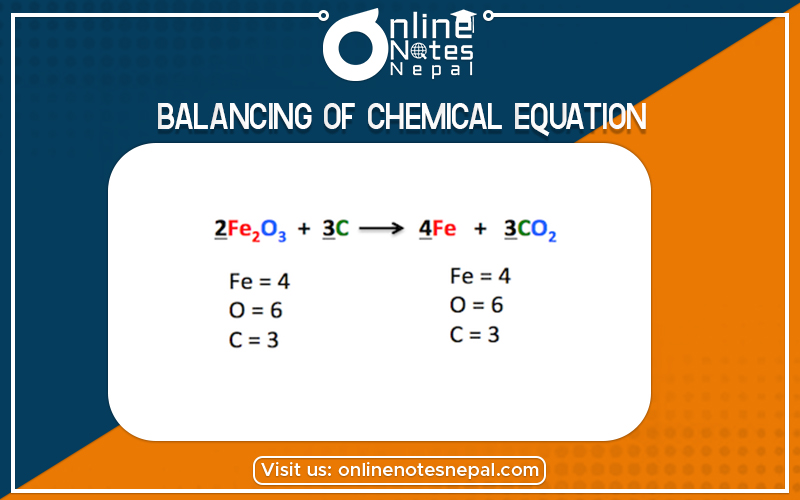
The balancing of chemical equation can be done by any of the following four methods:
1. By hit and trial method
2. By partial equation method
3. By oxidation number method
4. Ion-electron method
In this method, coefficient before the formulae or symbols of the reactants and products are adjusted in such a way that the total number of atoms of each element only both the sides become equal. This is called a material balance, or mass balance. In this method, first of all, atoms of the element which appears least in the chemical equation should be balanced. Then, the next one, and so only. Balancing of the chemical equation by hit and trial method is illustrated through the reaction which involves the burning of methane gas in the excess of air (or oxygen) to form carbon dioxide gas and water. The reactions is,
Methane + Oxygen (from the air) → Carbon dioxide + Water
(i) The skeleton equation for this reaction is,
CH4 (g) + O2 (g) → CO2 (g) + H2O (l)
(ii) The number of atoms of each element on both the sides of the arrow.
As the number of atoms of His and one on both sides is not equal, the skeleton equation is not a balanced chemical equation.
(iii) Inspection of the skeleton equation shows that both carbon(C) and hydrogen(H) occur twice, and oxygen(O) appears thrice. So, a start is made by balancing carbon or hydrogen atoms. Carbon is already balanced. There are 4 hydrogen atoms only the left side, and two hydrogen atom only the right side. So, hydrogen can be balanced by placing a coefficient of 2 before H2O on the right-hand side.
(iv) Now, there is one carbon and four hydrogen atoms only either side of the equation. Thus, carbon and hydrogen are balanced. In this partly balanced chemical equation, there are four oxygen atoms only the right-hand side, while there are two only the left-hand sides, by 2.
(v) The resulting equation is,
CH4 (g) + 2O2 (g) CO2 (g) + H2O (l)
The number of atoms of each element, only both the sides of the equation
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) are equal. Therefore, the above chemical equation is a balanced chemical equation.
In partial equation method the complex reaction is first of all split into simple equations, such a simpler equation is called a partial equation. Each partial equation is first of all balanced by hit and trial method and is multiplied by some suitable number, these partial equations are added like algebraic equations.