Published by: Nuru
Published date: 26 Jun 2021
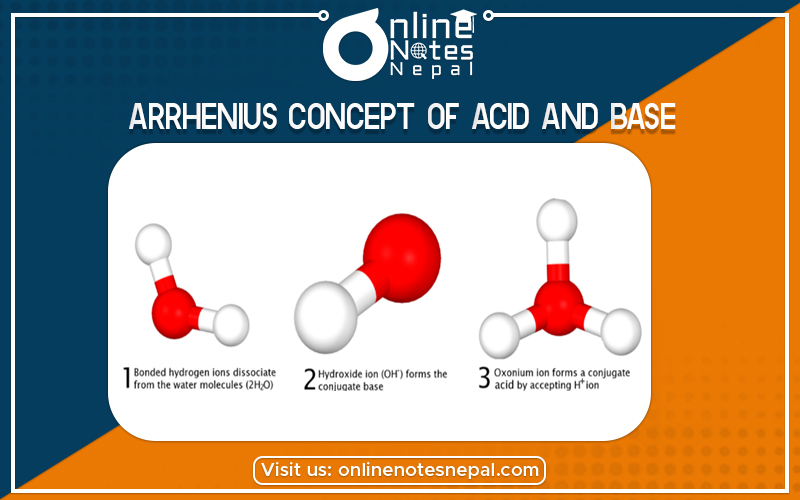
According to this concept, the compounds which can produce H+ Pons as the only positive ions when dissolved in water are called acids.
According to this concept HCL, H2SO4 and HNO3 are the acids because all of them produce Hydrogen ions when dissolved in water.
HCl (aq) H+ (aq) + CL– (aq)
H2SO4 (aq) H+ (aq) + HSO4– (aq)
HSO4– (aq) H+ (aq) + SO4– (aq)
According to this concept, the compounds which can produce Hydroxyl ion (OH ions) as the only negative ions when dissolved in water are called bases.
According to this concept, KOH, NaOH etc are the bases because all of them produce Hydroxyl ions when dissolved in water.
NaOH (aq) Na+ (aq) + OH– (aq)
KOH (aq) K+ (aq) + OH– (aq)
Mg (OH)2 (aq) Mg++ (aq) + 2OH– (aq)
AL(OH)3 (aq) AL+++ (aq) + 3OH– (aq)
Water-soluble bases are called alkalies so, all alkalies are bases but all bases are not alkalies.
Neutralization is the combination of H+ ions of acid and OH– ion of bases.
H+ (aq) + OH– (aq) H2O (l)